Case study: triangle theory - separation of a binary mixture
|
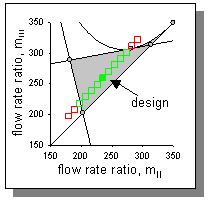
| Figure 1: Triangle theory according to M. Mazotti, G. Storti and M. Morbidelli (1997) for a i-pentane and 22DMB mixture. The grey area marks the region for complete separation. |
Separation of a binary mixture takes mainly place in the sections II & III of a TMB unit. The choice of the operation point is critical since the operation window for complete separation is rather small. The critical parameters are the flow rate ratios. The flow rate ratios are defined as the ratio of the net fluid/gas flow and the adsorbed phase flow. Already small changes in the critical flow rate ratios might result in incomplete separation. In order to determine an operation point for complete separation M. Mazotti, G. Storti and M. Morbidelli (1997) developed a so-called triangle theory, which characterizes the separability of the mixture depending on the flow ratio in the section II and III. This valuable design method is basis for the choice of the operation point. In our example we separate a mixture of 22DMB and i-pentane in a TMB unit packed with MFI crystals at a temperature of 473 K. We are using a multicomponent Langmuir isotherm with saturation capacities of 4 molecules per unit cell. The isotherm parameters were taken from Jolimaitre et. al. (2002) (bi-C5 = 4.1182x10-5 Pa-1 and b22DMB = 7.1179x10-5 Pa-1).
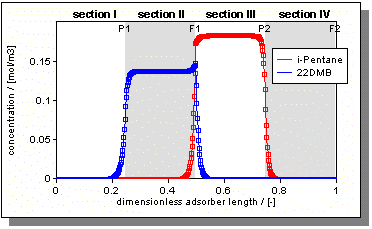 |
| Figure 2: Concentration profiles of the TMB unit in the gas phase. Note, the actual physical locations of z=0 and z=L coincide at the feed location of the desorbent. |
Applying Morbidelli and co-workers' method yields a region of possible flow rate ratios; see Figure 1. Our choice for this case study is mII=235 and mII=260. Note, the magnitude of the flow rate ratios are rather large due to the high values of the component's adsorptivities. Using the design equation proposed by Morbidelli and co-workers we can convert flow rate ratios, mj, to the interstitial gas velocities:
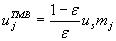
The total length of the TMB unit is 1 m. We chose 36 cm/h for the solid phase velocity in order to avoid high gas velocities in the sections. Furthermore, we do not account for an intra-particle voidage and the mass transfer resistances are negligible. The feed mixture contains 2 kPa i-pentane and 22DMB and the inert gas is only supplied in the second feed.
|
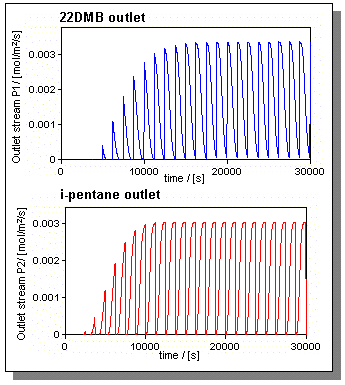
| Figure 3: Outlet concentration streams,uoutlet x ci,outlet,of a SMB unit separating i-pentane and 22DMB. |
| Section: | I | II | III | IV |
| Gas velocity [m/s]: | 0.06 | 0.03525 | 0.039 | 0.0225 |
Figure 2 shows the concentration profiles for the TMB unit at steady state. As can be seen, we obtain complete separation. In order to validate our model with the triangle theory we performed several simulations. The green symbols in Figure 2 represent simulations with complete separation, whereas red symbols denote runs with incomplete separation.
SMB unit
In practice one would apply a SMB rather then a TMB unit. Morbidelli and co-workers proposed conversion rules in order to determine the switching time, t*, and gas velocities in the sections,uSMB :
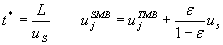
In our case study the gas velocities in a TMB unit are magnitudes larger then the velocity of the adsorbed phase. Hence, the final SMB gas velocities do not differ significantly from the ones chosen for the TMB unit. The switching time is around 2500 s; further details and an animation can be found here. The mixture is entirely separated. This can be clearly seen in Figure 3 where the composition flows in the withdrawals are plotted versus time.
Return to contents or next: simulated moving bed .
