Hexane isomerization in a SMB unit
Subsequently we consider the isomerization of n-hexane in SMB unit. From octane number consideration we wish to maximize the production of the di-branched isomer. Our analysis is restricted to a simplified reaction scheme.
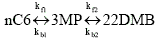
where the LH reaction rate expression for the two constituent reversible reactions are
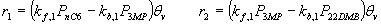
Note, the partial pressures in the rate expressions correspond to the fugacities and are obtained from the IAS theory. The pure components isotherms are described by a dual-site Langmuir isotherm; for details on the parameters see ' configurational entropy effects'.
The easier desorption of the product 22DMB, predicted by the rigorous LH-IAST approach, gives us a clue as to how we can exploit entropy effect to achieve supra-equilibrium conversions and improved selectivity. The selectivity is defined as the ratio of 22DMB to 3MP in the product. The trick is to adopt the simulated moving bed (SMB) reactor concept, where the desired 22DMB product is 'tapped' off at spatio-temporal positions where (when) it peaks. Since the 22DMB front moves ahead of the fronts of the other two species, the SMB concept offers the potential of not only improved nC6 conversion but also a higher 22DMB/3MP ratio in the product stream.
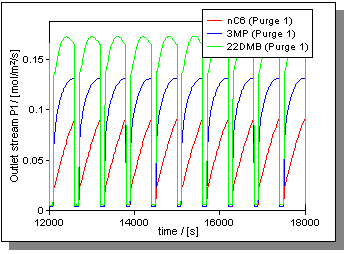
|
| Figure 3: Outlet composition stream. |
The simulations were carried out till true quasi-steady state conditions are achieved. Figure 2 shows the concentration profiles during two switches. We note that the 22DMB concentration is higher than that of 3MP and nC6 in the desorption zone. Further, we see that the 22DMB front moves ahead of that of 3MP and nC6 in the reactor segments between the feed and the withdrawal. As a consequence during the product withdrawal process, we have an almost pure 22DMB product shortly after a switch occurred. This explains high conversions at the beginning, which reduces to the equilibrium conversion value (75%) towards the end of the switching period; see Figure 3. For the same reason the 22DMB/3MP ratio is highest towards the start of the switching period and reaches the equilibrium value of 1.28 near the end.
