Chromatographic separation of hexane isomers using MFI zeolite
We investigate the breakthrough behaviour of a pulsed chromatograph, which is packed with MFI crystals. The fixed bed is assumed to be isothermal and operates at 362 K.
|
Temperature |
362.0 |
[K] |
|
Pressure |
6.895 |
[bar] |
|
Ugas |
0.07240 |
[m/s] |
|
Reactor length |
0.2 |
[m] |
|
Cat. voidage |
0.4 |
|
|
Cat. density |
1800 |
[kg/m3] |
|
Finite volumes |
40 / 20 |
bed/crystal |
|
DnC5/R2 |
1 |
[1/s] |
|
D3MP/R2 |
0.2 |
[1/s] |
|
D22DMB/R2 |
0.1 |
[1/s] |
|
DnC5/R2 |
5 |
[1/s] |
Our simulation of the pulsed chromatographic operation consists of three steps: (1) The zeolites are initially loaded so that the mixture loading is maintained at a loading higher than 4 molecules per unit cell. This is done by initially loading the sweeping component nC5 at a partial pressure of 36 kPa. The partial pressures of the other components are initialized with 5 Pa. (2) The mixture to be separated is injected through the chromatograph for a period of 25 s. The partial pressure of each component is 12 kPa. (3) After injection of the mixture, the feed is switched to pure n-pentane (that corresponds to a partial pressure of approximately 6.8 bar).
Sweeping the fixed bed is necessary in order to desorb the mixture from the zeolite as well as regenerate the bed. Note that a wide variety of other mixture and sweeping specifications result in a similar or even better separation of the mixture. The physical properties of the mixture compounds and sweeping compound have been previously listed ; see Table 1 in "configurational entropy considerations". The remaining input parameters for the simulation are specified in Table 1.
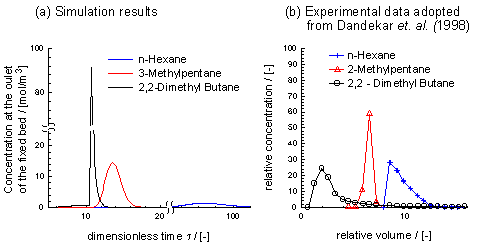
Fig. 1(a) shows the outlet concentration over time. The dimensionless time, t, is defined by t=t u/L, where t is the time, u is the initial interstitial gas velocity and L the reactor length. We notice from Fig. 1(a) that di-branched and mono-branched isomers are successfully separated. The di-branched component 22DMB is leaving the fixed bed first, followed by the mono-branched component 3MP and finally by n-Hexane. It is noteworthy that the results are also in agreement with the experiments of Dandekar et. al. (1998) for a similar setup; see Fig. 1(b).
Figure 1: (a) Predicted concentration at the outlet of the adsorber (b) Data of experiments separating an equimolar mixture of nC6, 2MP and 22DMB; adopted from Dandekar et. al. (1998).
Subsequently, we investigate the dynamic behaviour of the breakthrough in more detail. Fig. 2(a) shows the animation of the gas concentration profile along the bed length and Fig. 2(b)-(d) the intra-crystal fractional loadings at z/L= 0.0, 0.5 and 1.
Initial loading of fixed bed
At the beginning of the pulsed operation, virtually none of the mixture components are present in the zeolites. The zeolite crystals are exclusively loaded with nC5 (not shown in Fig. 2).Loading of the zeolite crystals
Once the mixture is injected into the fixed bed, the zeolite crystals are getting loaded. The differences in adsorption strength can be clearly seen at the inlet of the fixed bed; see Fig. 2 (b). 22DMB has the least adsorption strength due to configurational entropy effects and is almost "excluded" from the zeolite crystal. It only reaches a fractional loading of about 0.05. Hence, its gas concentration is comparably high and a significant fraction of 22DMB is carried downstream where it further adsorbs; see e.g. Fig. 2 (c). Note that downstream the adsorption strength is still limited due to the initial loading of nC5. In Fig. 1(a) (for 5 < t < 10) we see, that a small fraction of 22DMB is even leaving the fixed bed without being adsorbed at all.At the end of the loading phase, 22DMB was absorbed over the entire length of the fixed bed. Since the adsorption strengths of 3MP and nC6 are stronger, their spatial distributions along the fixed bed are narrower. 3MP is completely adsorbed in approximately the first tenth of the fixed bed, as indicated by zero gas concentrations for z/L > 0.1 in Fig. 2 (a). The spatial distribution of nC6 along the fixed bed is even narrower, as expected, due to its strong adsorption strength.
Further, we observe that no diffusional limitations are occurring during adsorption. The profiles of the fractional loadings are almost straight; see Fig. 2 (b-d). One should keep in mind that diffusional limitations might significantly influence the spatial distribution of loadings along the fixed bed.
Sweeping of fixed bed
The sweeping component nC5 is injected 25 s after the mixture was pulsed. The high partial pressure of nC5 and the strong adsorption strength ensure that the zeolites are loaded with the sweeping component and desorb the mixture components. As a result, we observe high driving forces when the sweeping component reaches the zeolite crystals. In order to illustrate this, we present in Fig. 3 the loadings and fluxes of a zeolite crystal at the inlet of the fixed bed.Figure 3: (a) fractional loadings and (b) calculated fluxes in the zeolite crystal at z =0 after sweeping component is injected.
At the end of the loading phase, the crystals are predominantly filled with the initially loaded nC5 and the high adsorbing component nC6. At this moment, only a small flux of nC6 is entering and a small flux of nC5 is leaving the crystals; see Fig. 3 at t = 25.0 s.
Already a fraction of a second later, nC5 is injected to sweep the fixed bed. Immediately, the flux of nC5 is increasing rapidly and displaces the mixture components in the zeolite; see Fig. 3 at t = 25.2 s. The negative fluxes of nC6, 3MP and 22DMB indicate that the components are desorbed. If we take a close look at the spatial loading gradients, we see that the loading of nC6 at the surface slightly increases, whereas the ones of 3MP and 22DMB rapidly drop. This behaviour is directly related to the adsorption isotherms and their "exclusion" of 3MP and 22DMB from the zeolites for high partial pressures of nC5 and nC6; see configurational entropy effects. Furthermore, we observe that nC6 is desorbed against its loading gradient. Such behaviour is called reverse diffusion and is caused by diffusional interaction between components (Krishna and Wesselingh,1997). Common models using Fick's law cannot capture such behaviour.
Figure 4: Concentration in the gas phase along the fixed bed while the sweeping component is injected (t > 25 s). The figure contains the same information as Fig. 2(a). Note that the time between snapshots is varying.
The rapid displacements of 3MP and 22DMB leads to vast increase of the concentrations at the inlet of the fixed bed. The resulting wave is carried downstream; see Fig. 4 and Fig. 2(a).
One might wonder why the wave peek for 22DMB gains during its journey and the one for 3MP declines. Reason for this is the spatial distribution of the loadings in the zeolites along the fixed bed. As discussed previously, 22DMB was adsorbed over the entire length of the fixed bed. Therefore, it is continuously desorbed now, causing a steady incline of the wave peek. In contrast to 22DMB, 3MP was only adsorbed in the first tenth of the fixed bed. As the wave passes this point, the MFI zeolites adsorbs 3MP before 3MP gets desorbed again; see Fig. 2(b-c). Hence, in the beginning of the sweeping, we observe a vast growing wave for 3MP, which afterwards declines and broadens. The same occurs to nC6. Since its absorption strength is much stronger, the wave becomes much smaller and broader than the one for 3MP. Note that the time between snapshots is varying.
Further details and reading can be found in Krishna and Baur (2003).
Return to contents or next: permeation of C1, C2, C3 and nC4 mixture across MFI membrane .